High-tech production of organs for transplantation is our future
Modern medicine has produced many kinds of high-tech miracles, among them gene therapy to correct malfunctioning genes, electrical stimulation devices to restore significant function after traumatic spinal cord injury, and surgery performed by robots.
Another medical area that desperately needs breakthroughs is transplantation of solid organs. We are making progress but are not quite there. Currently, donor organs — from a living donor or cadaver — must match the recipient’s tissue type and size, and often, they are not perfect. By one estimate, approximately half of transplanted organs are rejected by recipients’ bodies within 10-12 years, in spite of a constantly expanding understanding of what causes rejection.
Moreover, there is a severe shortage of organs. Currently, more than 100,000 Americans are waiting for transplants, and due to a shortage of hearts, lungs, livers, and kidneys, at least 17 die each day. However, two new approaches to providing organs for transplantation could ultimately both eliminate the need for organ donors and reduce the risk of tissue rejection.
Organ transplantation solution: 3D bioprinting
The first is three-dimensional (3D) bioprinting, which uses “bio-ink,” a printable material made from a patient’s own cells, to print layer upon layer, creating tissue that will not be rejected by the recipient. But in progressing from tissue to a complex organ, one critical challenge has been how to get blood to flow to keep the cells alive, and researchers have devised a number of approaches to this. They include threading tiny channels through the organ, where blood vessels develop when implanted in animals, or seeding channels with the endothelial cells that line the inside of blood vessels.
An exciting advance was reported earlier this year by a Swedish research group attempting to create human lungs by 3D printing. According to the lead author of the study, “We started small by fabricating small tubes, because this is a feature found in both airways and in the vasculature of the lung. By using our new bioink with stem cells isolated from patient airways, we were able to bioprint small airways which had multiple layers of cells and remained open over time.”
In addition to the daunting technical challenges, healthcare executive Daniel Troy has described the regulatory lassitude at the FDA that has discouraged commercial interest in the field. He observed that while the FDA is accustomed to evaluating the safety and efficacy of mass-produced therapies and medical devices, “bioprinted organs are one-of-a-kind creations, tailor-made for each patient,” and that the agency’s long-promised regulatory guidance for such products has not materialized.
Organ transplantation solution: genetic modification of pigs
A second approach to providing a sufficient supply of organs for transplantation is genetically engineering animals — most often, pigs — so that their transplanted organs will not be rejected. In effect, it uses genetic engineering to grow “humanized” tissues and organs in animals. There was a breakthrough with this approach in 2018, when scientists used gene editing to create hybrid embryos containing both human and sheep cells.
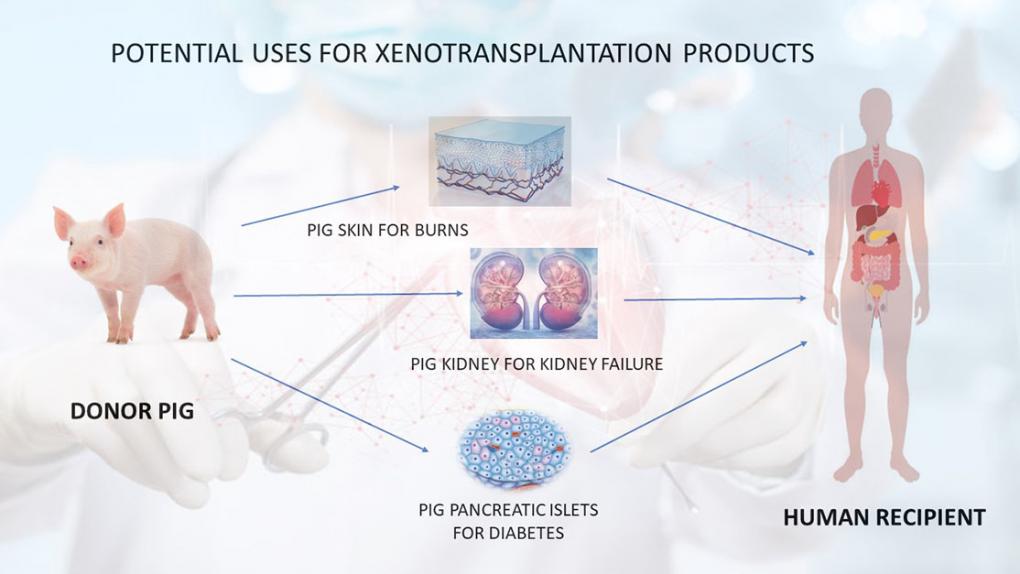
Another milestone occurred in December 2020 when the FDA “approved a first-of-its-kind intentional genomic alteration (IGA) in a line of domestic pigs” called GalSafe, which may be used for food or human therapeutics. The IGA in the animals eliminates the gene that makes α-Gal, a sugar molecule found naturally on the surface of porcine cells. It is the source of allergy in some people when they consume certain meats, and it also is involved in tissue or organ rejection after transplantation into humans. That was the first IGA in an animal approved by the FDA for both human food consumption and as a potential source for therapeutic uses.
There is considerable research underway to create lines of pigs for transplantation, but there is also controversy about the extent of genetic modification that will be necessary to both avoid rejection and ensure the absence of harmful pathogens. Therefore, it will be interesting to see at what stage of research and which genetic constructions regulators allow for the first clinical trials.
The FDA unveiled in 2018 the Plant and Animal Biotechnology Innovation Action Plan, which includes a pilot project — the Veterinary Innovation Program — similar to the breakthrough designation for drugs that is supposed to offer guidance and assistance by FDA experts to sponsors seeking approval of animal cell-based or biotech products.
We shall see whether those promises are translated into actions. The FDA’s record on animal biotechnology has been far from enviable. There is an urgent need for organs for transplantation. Regulators must get this right and without delay.
Henry I. Miller, a physician and molecular biologist, is a senior fellow at the Pacific Research Institute. He was the founding director of the FDA’s Office of Biotechnology. Find Henry on Twitter @henryimiller.
Read More
