The Biosimilar Opportunity: Quarterly Update
In October 2019, the Center for Medical Economics and Innovation at the Pacific Research Institute, under the direction of Dr. Wayne Winegarden, released its second study documenting the savings potential enabled by biosimilars. Biosimilars are medicines manufactured in, or derived from, biological sources that are developed to be similar to FDA-approved reference products. Biosimilars are only approved to compete in nine biologic drug classes in the U.S. and are available in seven of these drug classes currently. Europe, for comparison, has approved more than three times as many biosimilars as the U.S. Another problem, except for the Filgrastim drug class (the biosimilar Zarxio), biosimilars have not gained a meaningful share of the U.S. market where they are available.
Despite these obstacles, the October 2019 study found that biosimilars still generate over $240 million in total savings. If biosimilars grew to 25 percent, 50 percent, and 75 percent of the market, the potential savings would be $2.4 billion, $4.7 billion, and $7.0 billion, respectively based on data through the beginning of 2019. Due to the large potential savings, solving the obstacles preventing wider adoption of biosimilars is imperative.
Updating the analysis, this Issue Brief projects the potential savings that wider use of biosimilars can generate for 2020. These projected savings are based on the volume data through March 2020 and pricing data that are valid through 2020 Q1. To ensure consistency with future updates, the savings potential for each biologic drug class is calculated relative to each originator biologic’s price prior to biosimilar entry. As a result, the potential savings will be larger than the original estimates since these estimates also capture the savings created when the originator biologics lower their prices in response to greater biosimilar competition.
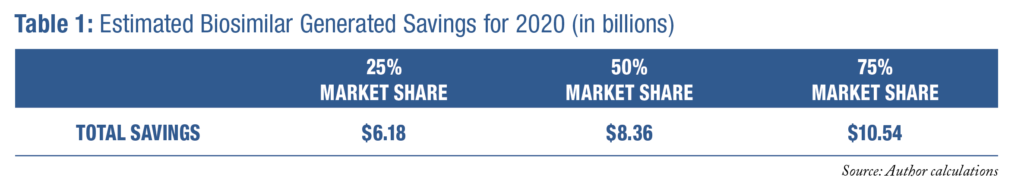
Table 1 demonstrates that the total potential savings could be as high as $10.5 billion in 2020, if biosimilars were able to obtain 75 percent market share. If biosimilars represented 25 percent of the biologics market in these 9 drug classes, which would still be a large increase, the potential savings in 2020 would be $6.2 billion. However, without effective policy and market reforms, it will be difficult for the healthcare system to realize these large potential savings that biosimilars offer.