The Biosimilar Opportunity: Quarterly Update No. 4 – October 2021
In October 2019, the Center for Medical Economics and Innovation at the Pacific Research Institute released its second study documenting the savings potential enabled by biosimilars. Biosimilars are medicines manufactured in, or derived from, biological sources that are developed to be similar to FDA-approved reference products. Biosimilars are approved to compete in nine biologic drug classes in the U.S. and are available in seven of these drug classes currently.
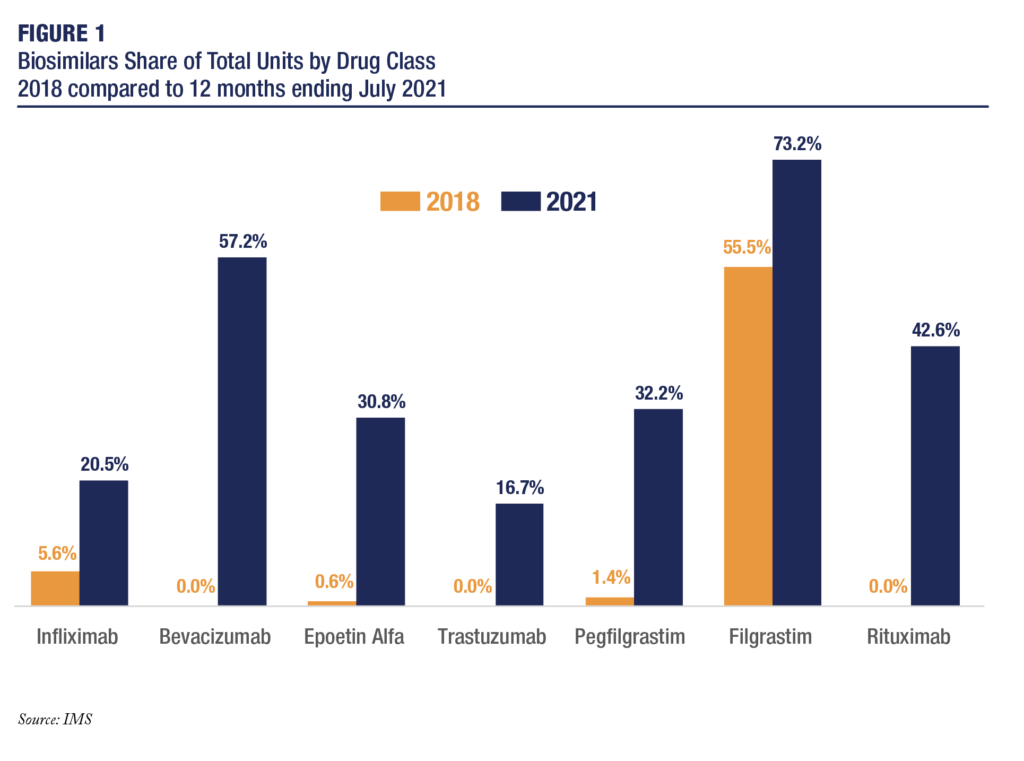
Since 2018, biosimilars’ market share has grown appreciably, see Figure 1. Thanks to this significant growth and based on the methodology used in the October 2019 analysis, total 2021 savings are on pace to reduce total expenditures by $7.8 billion compared to the prices that existed prior to the introduction of biosimilar competition.
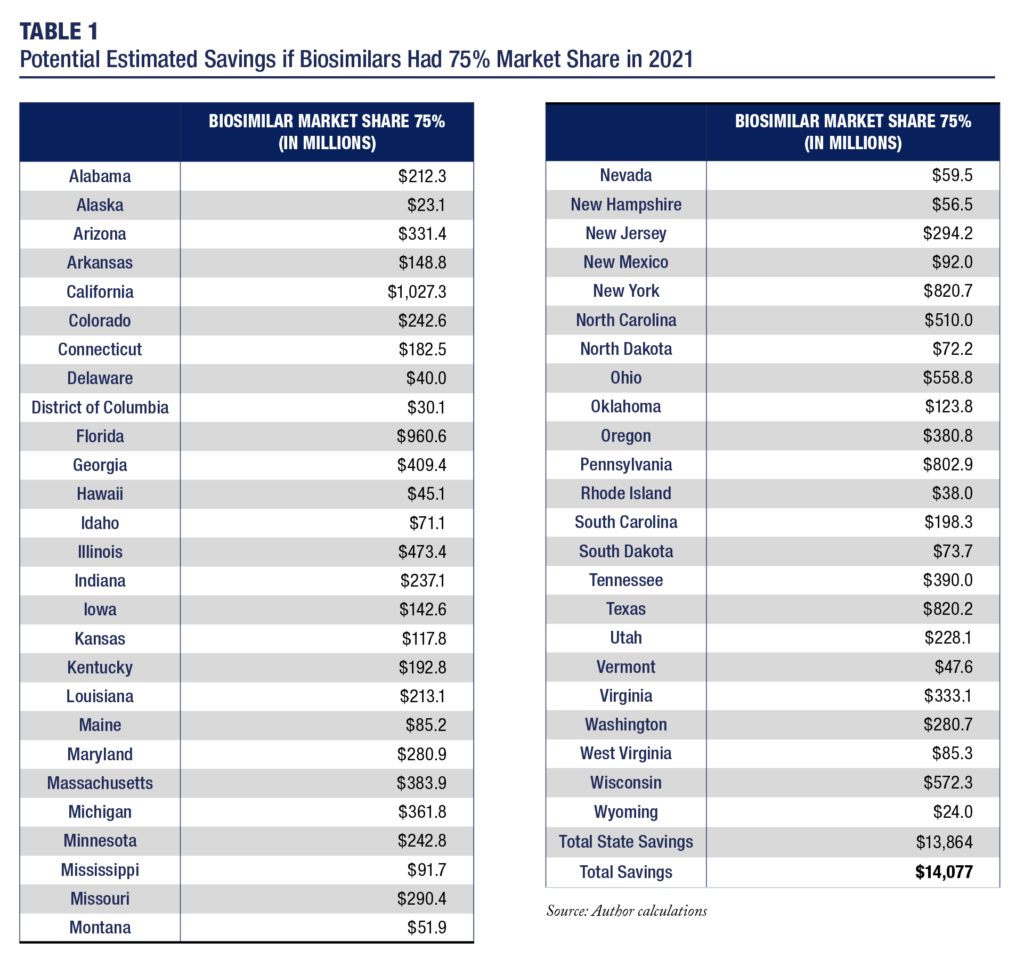
The potential savings are even greater. Should biosimilars grow to 75 percent of the market, which is still less than the share of the market for generic medicines in the U.S. or for biosimilars in many EU countries, the total potential savings in 2021 could be as high as $14.1 billion, see Table 1. Table 1, which also displays the savings potential by state, calculates the savings based on the 12-month volume data through July 2021 and pricing data that are valid for the second quarter of 2021.
Table 1 demonstrates that increased biosimilars use will generate even larger systemic savings that will meaningfully reduce the drug affordability problem. Importantly, these reduced expenditures would be associated with an approximately $814 million reduction in patient out-of-pocket costs. Therefore, greater use of biosimilars offer both large systemic savings and large direct savings to patients. However, effective policy and market reforms are essential for the healthcare system to realize this savings potential.
Endnotes:
- Winegarden W (2019) “Incenting Competition to Reduce Drug Spending: The Biosimilar Opportunity” Pacific Research Institute: Center for Medical Economics and Innovation, July; https://www.pacificresearch.org/wp-content/uploads/2019/07/BiosimilarsCompetition_F.pdf. Winegarden W (2019) “The Biosimilar Opportunity: A State Breakdown” Pacific Research Institute: Center for Medical Economics and Innovation, October; https://www.pacificresearch.org/wp-content/uploads/2019/10/BiosimilarSavings_web.pdf.
- The price data are the average sales price data from the Centers for Medicare and Medicaid Services (https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2021-asp-drug-pricing-files). Readers interested in an in-depth description of the data and sources should refer to the original studies.

